http://www.autovaccine.de
imprint (in German language)
Therapeutic autogenous vaccination (homologous autovaccination) for the treatment of chronic or recurrent bacterial infections.
|
Important note:
|
|
|
|
This site provides scientific information and results, only. The information given on this site or on any site of this domain must not be regarded as recommendation for treatment or as diagnostic or therapeutic advice.
All information about manufacturing process, distribution, pricing, and application are for Germany, only.
|
|
|
|
|
|
|
|
Definition: Autovaccines/autogenous vaccines
Autogenous vaccines (formerly known as autovaccines) are therapeutic vaccines, individually tailored for a patient. These vaccines are made from cultures of pathogenic micro-organisms which are isolated from the site of an infection (furuncle, boils, abscess, urine). Because of long manufacturing times (up to four weeks) autogenous vaccines are only useful in case of chronic or recurrent disease but not for the treatment of acute diseases. Specificity of these tailored vaccines is an important characteristic of autogenous vaccines. Following the original protocol provided by Sir Amlroth WRIGHT in 1903, autogenous vaccines are heat inactivated suspensions of bacterial cultures where the micro-organism is obtained from a lesion or from the site of infection of that patient for which the autogenous vaccine is ought to be manufactured. The autogenous vaccine is therefore in certain way of therapeutic action and as the time a prophylaxis against further recurrent episodes. The immune system of the patient is supposed to be modulated in order to be able to prevent future relapses.
The most prominent indications were autogenous vaccines are prescribed are Staphylococcus aureus induced furunculosis (boils, abscesses, deap seated cutaneous infections), urinary tract infections, secondary infections of diabetic ulcer (Staphylococcus aureus MRSA, Pseudomonas aeruginosa, and others).
Manufacturing process of autogenous vaccines
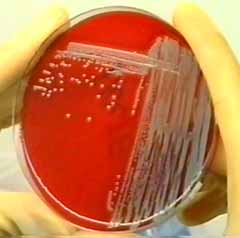
In a first step, micro-organism are cultured from swabs or tissue specimens, obtained from a site of infection (e.g. pus of a furuncle). The culture micro-organism (for instance the Staphylococcus aureus shown below on the left) will than be suspended in sterile, pyrogen free saline. There are different protocols in use, however, they all have in common that the suspended micro-organisms are inactivated, either by use of chemicals or by use of heat. The inactivated whole cell autogenous vaccine is bottled in a series of eight ot more vials. Each vial is one injection, injections are made in intervalls of several days or weekly (depending on the protocol recommended by the manufacturer)
 |
 |
 |
Staphylococcus aureus culture on standard sheep blood agar (columbia agar) provides the basis for autogenous vaccine manufacturing.
|
vial with one dose of autogenous vaccine (clear vials are used for demonstration only).
|
set of 10 vials for subcutaneous application. The elevnth vials is for control purpose, only. The actual number of vials/injections may vary depending on the manufacturer.
|
|
|
|
(all images: SWR3). |
|
|
|
|
|
|
Time required for manufacturing
Manufacturing of autogenous vaccines is a time consuming process. Typically, up to four weeks are needed until a final product is distributed. The isolation of a micro-organism from a clinical specimen may take up to three days (including identification of the micro-organism). Heat inactivation and subsequent sterility testing require a minimum of two weeks. The tested and QC passed autogenous vaccine is than send to a pharmacy.
Physician's qualification
In principle, every physician is qualified to administer autogenous vaccines. However, even if patients are familiar with injection/puncture techniques self treatment is strictly prohibited. Only a physician is allowed to administer!
Therapeutic effect of autogenous vaccines
Since introduction of autogenous vaccines into human medicine some 100 years ago it has been reported frequently that certain types of recurrent infections can be succesfully treated with autogenous vaccines. This is in particular true for furunculosis and deep seated cutaneous abscesses caused by Staphylococcus aureus. However, at this time clinical studies to test for the efficacy of autogenous vaccines are lacking.
Autogenous vaccines are not only personalized but individualized therapeutics as they are manufactured from a micro-organism isolated from the patient for whom the autogenous vaccine is going to be manufactured. Due to the individual nature of autogenous vaccine no general statement about the observed therapeutic effect can be given.
Mode of action
No scientific reports are available explaining the mode of action of autogenous vaccines. In the beginning of the 20th century the so-called opsonic index was used to determine the influence of autogenous vaccination on the patient's immune system. This opsonic index suggested that antibodies are induced by autogenous vaccination. However, measurements in some laboratories failed to clearly demonstrate an increase in specific antibodies after treatment. It has been suggested that autogenous vaccination may activate parts of the innate immune system but this has to be figured out.
Indications
As mentioned earlier autogenous vaccines are not useful in case of acute diseases. The classical indication which have been reported in the literature are:
- furunculosis (abscesses, boils) when chronic or recurrent over a longer period of time
- secondary infections of diabetic ulcer
- urinary tract infections
- periodontitis (depending on the micro-organism responsible and the severity of disease)
- adjunct therapy to antimicrobial treatment in case of osteomyelitis (see next paragraph)
Autogenous vaccines are NOT useful in case of viral infections, infections caused by spirochetes (borreliosis, syphilis) and in general in non-infectious conditions.
Autogenous vaccination for the treatment of chronic osteomyelitis
A number of scientists and physicians have dealt with the use of autogenous vaccine for the (adjunct) treatment of chronic osteomyelitis.
The work of BOLOCZKO S. & BLADOWSKI K. (1994): [Autovaccine used in comprehensive treatment of staphylococcal inflammation of the bone. Med. Dosw. Mikrobiol. 46(1-2 Suppl.):51-57 (article in Polish)] and from the group of DEVITO in Bari (Italy) have demonstrated that autogenous vaccination might be beneficial, reducing the rate and severity of recurrent episodes of the diesease.
Work conducted at the University of Stettin/Szczecin (KALEMBA, unpublished) suggests, however, that autogenous vaccine treatment in case of Staphylococcus aureus caused osteomyelitis has the highest efficacy in the early states of disease. With disease duration the efficacy seems to decline significantly.
Autogenous vaccines - current knowledge
Autogenous vaccines have been introduced into human medicine more than 100 years ago. The bacteriologist and immunologist A.E. Wright reported the use of autogenous vaccine in the treatment of boils in 1903. Beginning 1907 a number of case reports were published. In addition, improved protocols for autogenous vaccine manufacturing and extended indications were reported in the scientific literature. Until now more than 650 specific publications have been recorded, with a peak in the 1930's (list of references). This publication activity implies that autogenous vaccines, their use and efficacy have been studied at least on an empirical basis. However, specific scientific work pointing towards the mechanism of action of autovaccines are limited and no accepted model about how autogenous vaccines influence a patients' immune system is available.
The history of autogenous vaccination?
As mentioned above, autogenous vaccines are in use in human medicine since the eraly 20th century. By clicking the thumbnail below you reach a graph showing the publication statistics as revealed by searching the medline.

What is known about the safety?
Autogenous vaccines are in general as safe as other vaccines. However, each vaccine and each medication carries the risk of unwanted or adverse effects. The same holds true for autogenous vaccines, too. Quite normal is a mild local reaction at the site of injection. This mild redness and swelling may be interpreted as an indication of activation of the immune system. One serious side effect, a case of systemic inflammatory response syndrome (SIRS) was reported in a timely association of autogenous vaccine use (vs. S. aureus; WOJTACHA, A. et al (2002); Clin. Pract. Rev. 3:28-30). However, no evidence is provided that the SIRS was indeed caused by the autogenous vaccine.
In principle patients and physicians should pay particular attention to local or systemic reaction which in association with autogenous vaccine treatment. If, however, severe reactions occur, the treatment must not be continued. As a rule autogenous vaccine must be given by a physician! Following application, the patient should stay under observation for an additional hour.
|



